You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences.
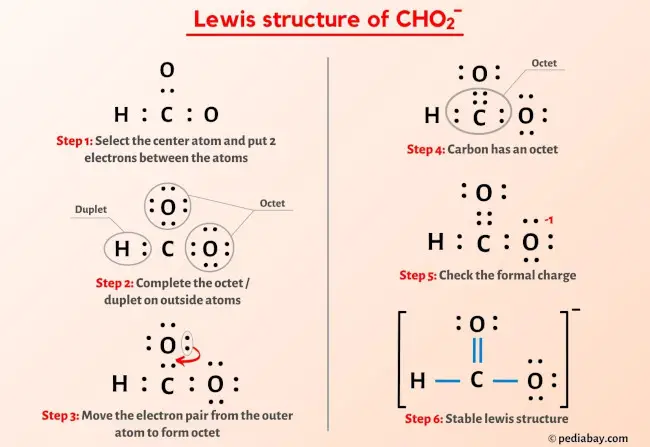
CHO2- Lewis Structure in 6 Steps (With Images)
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen’s valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks

Source Image: remedialeducationpoint.com
Download Image
Answer link. Carbon atoms have 4 valence electrons each. To find how many valence electrons are in an atom, you can look at the periodic table: Look at the writing above each Group, or column. The number next to the “A” is the number of valence electrons in an atom of an element in that Group. Carbon is in Group 4A, so it has 4 valence electrons.
![Explained] How Many Valence Electrons Does a Conductor Generally Have? - Circuits Gallery](https://www.circuitsgallery.com/wp-content/uploads/2023/11/Valence-Electrons.webp)
Source Image: circuitsgallery.com
Download Image
ChemQuest 29 | PDF | Valence (Chemistry) | Chemical Bond
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.

Source Image: m.youtube.com
Download Image
How Many Valence Electrons Does A Carbon Atom Have
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
How many valence electrons does carbon have?||How to find Valence electrons for carbon(C) – YouTube
Steps for Writing Lewis Structures. Example \(\PageIndex2\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2.
WebElements Periodic Table » Cobalt » properties of free atoms

Source Image: webelements.com
Download Image
The number of electrons, present in the valence shell of carbon of carbanion bearing – YouTube
Steps for Writing Lewis Structures. Example \(\PageIndex2\) 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2.

Source Image: youtube.com
Download Image
CHO2- Lewis Structure in 6 Steps (With Images)
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences.
Source Image: pediabay.com
Download Image
ChemQuest 29 | PDF | Valence (Chemistry) | Chemical Bond
Answer link. Carbon atoms have 4 valence electrons each. To find how many valence electrons are in an atom, you can look at the periodic table: Look at the writing above each Group, or column. The number next to the “A” is the number of valence electrons in an atom of an element in that Group. Carbon is in Group 4A, so it has 4 valence electrons.

Source Image: scribd.com
Download Image
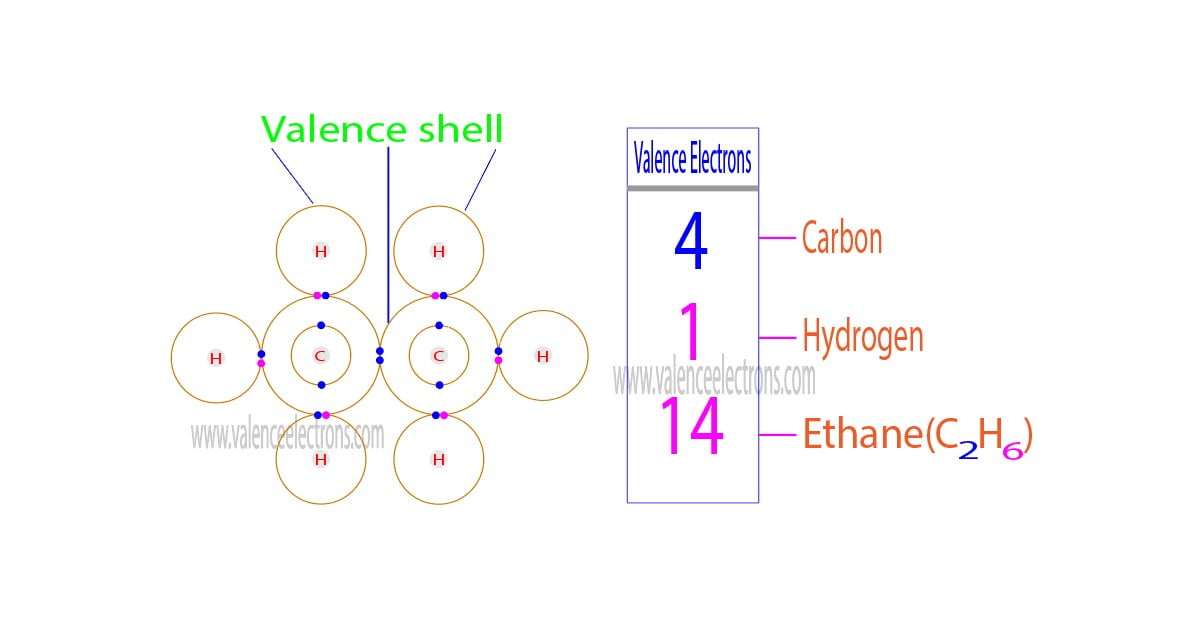
How to Find the Valence Electrons for C2H6 (Ethane)?
Four covalent bonds.Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both
Source Image: valenceelectrons.com
Download Image
Lewis Electron Dot Structures – Counting Valence Electrons
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”.
Source Image: physicsclassroom.com
Download Image
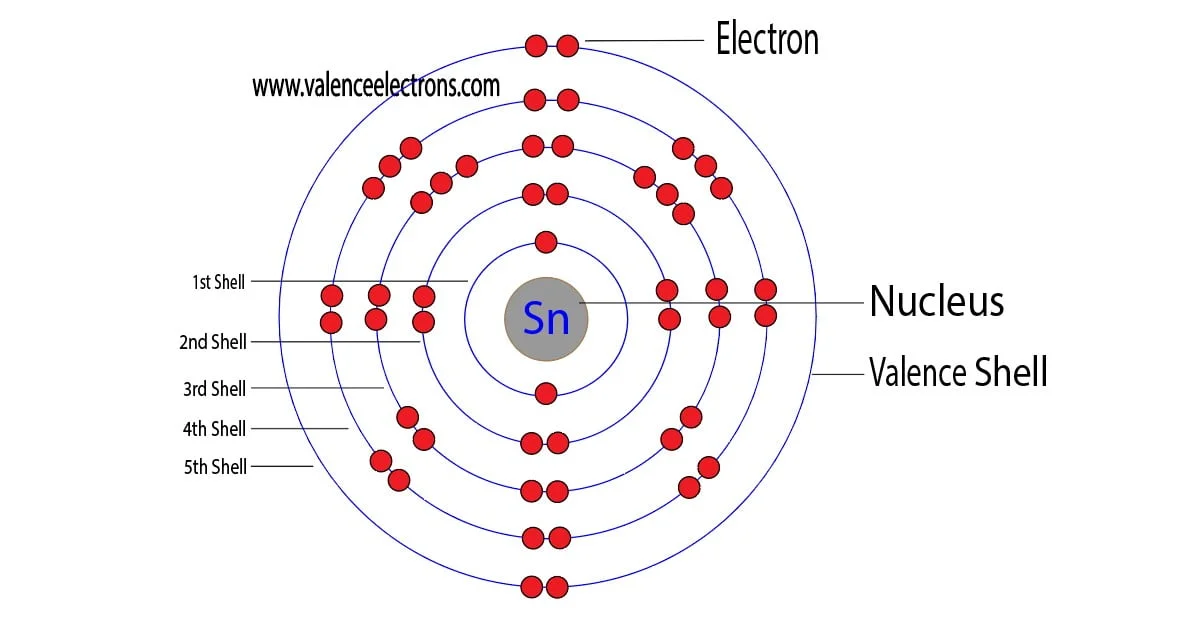
How to Find the Valence Electrons for Tin (Sn)?
Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
Source Image: valenceelectrons.com
Download Image
The number of electrons, present in the valence shell of carbon of carbanion bearing – YouTube
How to Find the Valence Electrons for Tin (Sn)?
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen’s valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
ChemQuest 29 | PDF | Valence (Chemistry) | Chemical Bond Lewis Electron Dot Structures – Counting Valence Electrons
Four covalent bonds.Carbon has four valence electrons and here a valence of four. Each hydrogen atom has one valence electron and is univalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both